1. Synthesis of nanomaterials
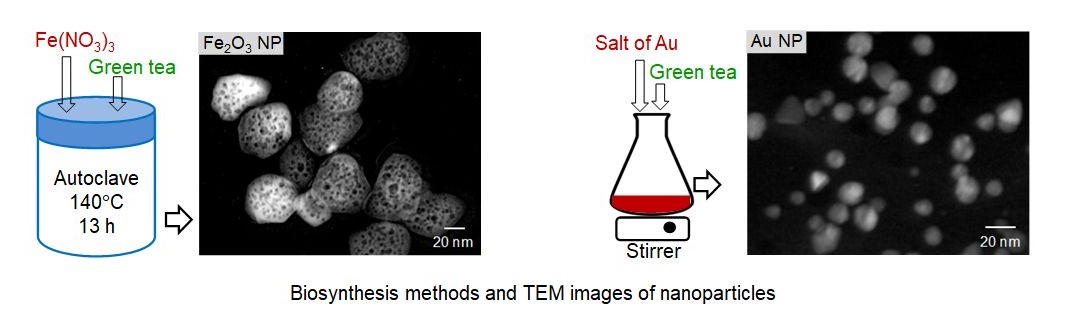
Nanostructured materials show unique physical and chemical properties that differ from their bulk materials. This research aims to synthesize new nanostructured materials and to explore their properties. We synthesize different nanostructured materials with new shapes, sizes, and porosities by using conventional or modified methods. We also work to develop "green synthesis" routes for nanomaterials synthesis. Figures below show biosynthesis routes (developed in our lab) for iron oxide (Fe2O3) and gold (Au) nanoparticles together with their TEM images. We study physical (optical, magnetic) properties and chemical (catalytic) properties of the nanomaterials followed by their applications in electronic devices, catalysis, wastewater treatment, biodegradable plastic, etc.
2. Solar energy conversion
Solar energy is a vast and inexhaustible resource from the sun that can be converted into chemical, electrical or heat energy. Our group works for the efficient conversion of this infinite energy source into chemical (hydrogen) energy and electrical (solar cell) energy by using nanotechnology.
(a) Solar to chemical energy conversion
Solar energy can be converted into chemical energy (hydrogen energy) via
water splitting by using a semiconductor photocatalyst. In a photocatalytic
reaction, water molecules are oxidized by photoholes at the valance band
of a semiconductor photocatalyst to form O2(g) and H+. The protons (H+) are then reduced by photoelectrons at the conduction band of photocatalyst
to produce H2(g). H2.generation increases when a co-catalyst (generally, metal nanoparticles,
such as Pt ) is used with the photocatalyst (see figure below). To develop
a highly active photocatalytic system, we synthesize nano photocatalysts
of different sizes and shapes, core-shell type nano structures or nanocomposite
materials and evaluate their performances in terms of H2.production. We also apply photoactive biomolecules (extracted from bacteria)
and natural clay (graphite silica) together with the nano photocatalysts
and study their mechanisms of activities to further improve the efficiency
of hydrogen production. SEM images of some photocatalysts, synthesized
in our lab, are shown in the figure below.

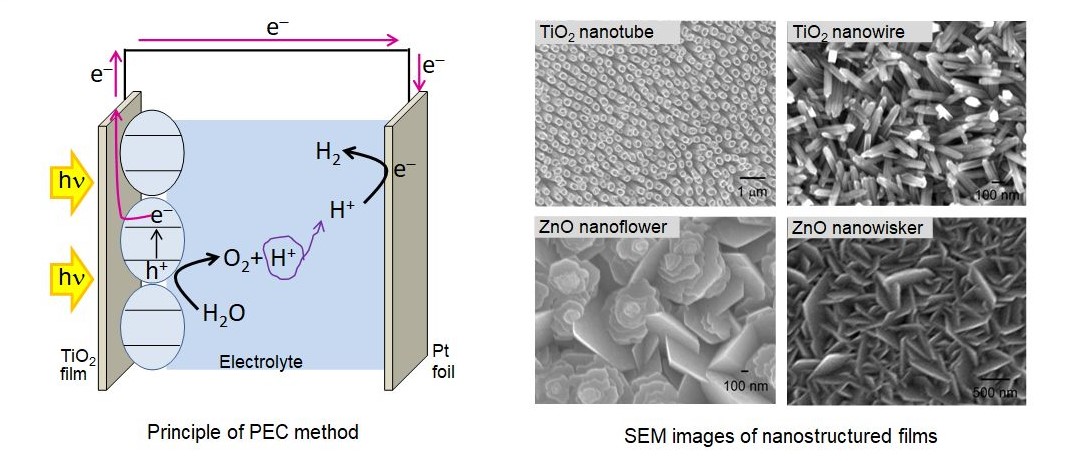
Photoelectrochemical reaction is another method of hydrogen production via water splitting. In this method, a semiconductor photocatalyst film is used as a photoanode. Photoholes at the anode react with water molecules to produce O2(g) and H+. Photoelectrons from the anode are transferred to the cathode through an external circuit where H+are reduced to produce H2(g). We fabricate and apply self-aligned 1D and 2D nano structured thin films (see figure below) of various semiconductor oxide materials such as ZnO, TiO2,.Fe2O3.etc., which provide a large surface area and improved charge collection efficiency. Moreover, nanoparticles of CdS, CdSe, Ag, ZnS, amorphous TiO2,.SiO2.etc. are used with these films to achieve improved activities under the visible light irradiation or/and improved charge separation.
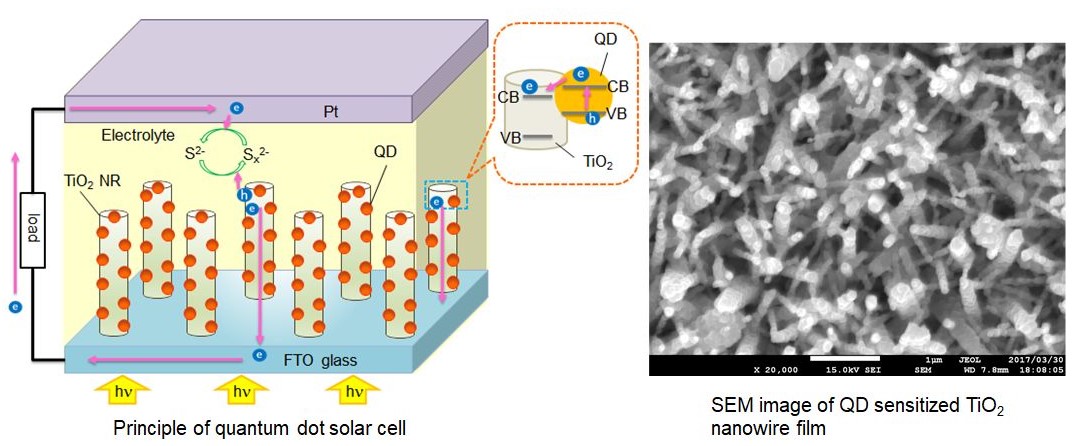
(b) Solar to electrical energy conversion
The aim of this work is to fabricate a low-cost solar cell, which could
replace the expensive Si solar cell. Quantum dots (QDs) solar cell is considered
to be one of the potential candidates to replace Si solar cell. QDs are
the tiny particles or nanocrystals of semiconductor materials with diameters
in the range of 2-10 nm and a central theme in nanotechnology. Due to their
unique electronic properties and multi exciton generation effect, the efficiency
of QDs solar cell is predicted to be ~70%, which is nearly three times
higher than that of conventional Si solar cell. However, the maximum efficiency
of QDs solar cells reported so far is 5%. We work to improve the efficiency
as well as the stability of QDs solar cells by increasing charge collection
and charge separation. For the effective charge collection, we use 1D nanostructured
films (instead of nanoparticles film) of TiO2.or ZnO in a QDs solar cell. Moreover, Ag nanoparticles and ALD (atomic
layer deposition) deposited amorphous TiO2.or SiO2.coating is used for the efficient charge separation. The SEM image, in
the figure below, shows CdS QDs deposited TiO2.nanowire film fabricated by hydrothermal and SILAR methods.
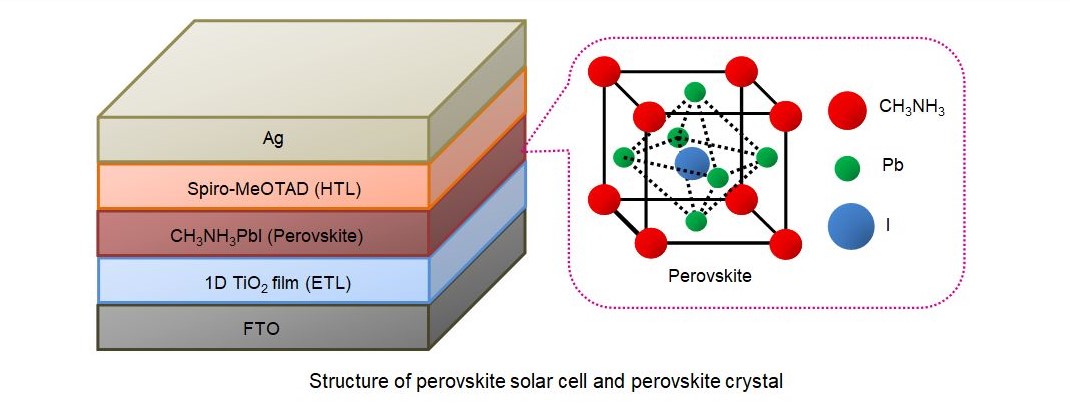
Perovskite is an organometallic compound, which is presently the most attractive material in the field of organic or organic-inorganic heterojunction solar cells. It is also a potential candidate for low-cost solar cells. In collaboration with Hirose lab, we are working to improve the performance and stability of perovskite solar cells by improving the crystallization of perovskite and effective charge separation. We use 2-steps spin-coating or vapor assisted deposition for the formation of perovskite films. The structure of a perovskite solar cell is shown in the figure below (ETL : Electron transport layer, HTL : Hole transport layer).
3. Smart Nano Materials
Smart materials have one or more properties that react to changes in their environment. This means that one of their properties can be changed by an external condition, such as temperature, light, pressure, electric or magnetic field. This change is reversible and can be repeated many times. Our lab is promoting research and development on the following two types of smart materials.
(a) Multiferroic Materials
Multiferroics are materials in which magnetism and ferroelectricity coexist.
Strong magnetoelectric (ME) coupling in these materials allows for electric-field
control magnetic properties, as well as magnetic field tuning of electrical
polarization (see the illustration below). Besides a scientific interest
in their physical properties, multiferroics have potential for applications
as actuators, switches, magnetic field sensors or new types of electronic
memory devices with ultra-low energy consumption. One of the most promising
multiferroic material is Bismuth Ferrite (BiFeO3). In our lab, we synthesize various metal-doped BiFeO3 micro or nano materials and study their magnetic and ferroelectric properties.
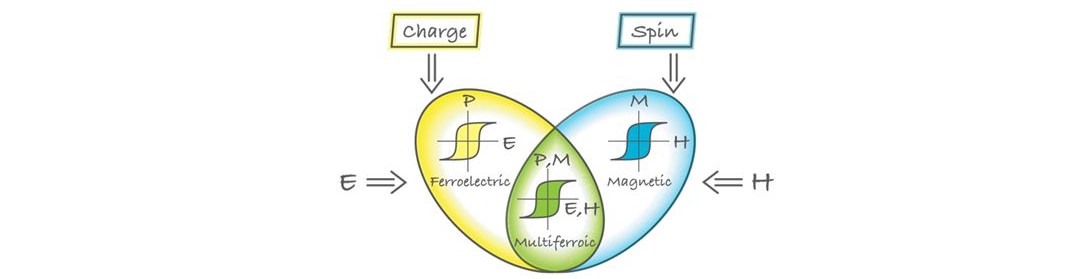
Combination of ferroelectric and (ferro) magnetic properties in multiferroic materials (Illustraion:Alan Stonebraker )
(b) Magnetic Semiconductor
Magnetic semiconductors are semiconductor materials that exhibit both
ferromagnetism (or a similar response) and useful semiconductor properties.
Among various magnetic semiconductor materials, Gd2S3 has a comparatively small bandgap (2.95 eV). We synthesize nanoparticles
of Gd2S3 and study their optical and magnetic properties for possible applications
in energy devices.
